 |  | |||||||||||||||||||||||||||
 What is Tungsten
Minerals and Deposits
Tungsten Uses
Tungsten Conversion Measurements
APT Pricing Charts
International Tungsten Industry Association
| Tungsten, (or Wolfram as it is known in many parts of the world) is an extremely hard and very dense gray to white metallic element extracted from wolframite, scheelite and other minerals. Tungsten has the highest melting point, lowest coefficient of expansion and lowest vapor pressure of any metal. It is also corrosion resistant and does not break down or decompose. Due to its unique attributes tungsten has few, if any, replacements in a majority of its industrial applications.
Minerals and Deposits Tungsten occurs in nature only in the form of chemical compounds. Although more than thirty tungsten bearing minerals are known, only two of them are important for industrial use, namely wolframite and scheelite.
Pure scheelite has blue-white fluorescence in ultraviolet light, a property which is utilised in prospecting. Wolframite is a general term for iron and manganese tungstates where the iron/manganese ratio can vary. A mineral with more than 80% FeWO4 is called Ferberite and a mineral with more than 80% MnWO4 is called Hübnerite. All tungsten deposits are of magmatic or hydrothermal origin. During cooling of the magma, differential crystallization occurs, and scheelite and wolframite are often found in veins where the magma has penetrated cracks in the earth's crust. Most of the tungsten deposits are in younger mountain belts, i.e. the Alps, the Himalayas and the circum-Pacific belt. The concentration of workable ores is usually between 0.3% and 1.0% WO3 Tungsten Uses 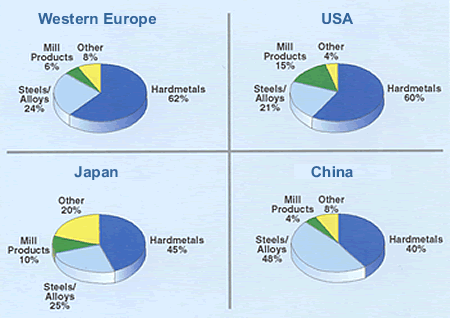 Source: USGS, 2007 Ammonium Paratungstate (APT) APT [(NH4)10[H2W12O42] · 4 H2O] is the main intermediate and also the main tungsten raw material traded in the market. APT is usually calcined to yellow (WO3) or blue oxide (WO3-X; a slightly substoichimetric trioxide with varying oxygen content). Tungsten Metal Powder (W) Yellow or blue oxide is reduced to tungsten metal powder by hydrogen. The reduction is carried out either in pusher furnaces, in which the powder passes through the furnace in boats, or in rotary furnaces, at 700-1,000°C. Tungsten Carbide (WC) Most of the tungsten metal powder is converted to tungsten carbide (WC) by reaction with pure carbon powder, e.g. carbon black, at 900 - 2,200°C in pusher or batch furnaces, a process called carburization. Tungsten carbide is, quantitatively, the most important tungsten compound. Because of its hardness, it is the main constituent in cemented carbide (hardmetal). 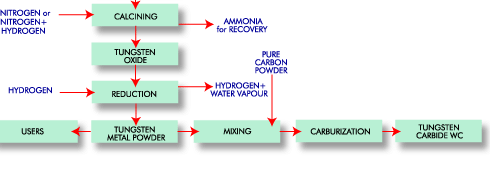 Tungsten Conversion Measurements
Tungsten Conversion Measurements
|
| Disclaimer |